Medical Pharmacology Chapter 35 Antibacterial Drugs
Penicillins And Others
Beta-lactamase inhibitors
 |
Overview
Tazobactam exhibit the broad spectrum of activity against many Ambler Class A β-lactamases, including:
TEM-1: TEM-1 has been identified as the most common plasma-encoded beta-lactamase in Gram-negative bacteria (class A enzyme).3
"TEM" was so named because the isolate was obtained from a fecal culture of an Athenian patient named Temoniera in 1963.6
SHV-1: SHV-1 Is a class A beta-lactamase with a spectrum of activity similar to TEM-1 but with better activity against ampicillin.4
SHV-1 has been found in several species of enterobacteria.
The SHV-1 beta-lactamase has been found at high frequency (up to 80%-90%) in Klebsiella pneumoniae.4
and a wide range of clinically important ESBLs.
Extended-spectrum β-lactamases (ESBLs) are described as are rapidly evolving beta-lactamase group sharing ability to hydrolyze third-generation cephalosporins and aztreonam but remain sensitive to inhibition by clavulanic acid.5
ESBLs represent mutations from genes for TEM-1, TEM-2 and SHV-1.5
Tazobactam does not exhibit activity against Class B or most Class C enzymes.
Mechanism of Action
 |
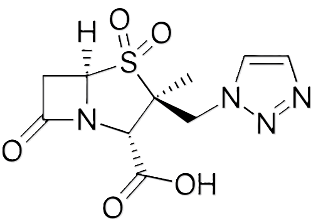
Tazobactam is a synthetic penicillanic acid sulfone, structurally related to sulbactam.
Tazobactam contains a 1,2,3-triazole side chain on the beta-lactam ring.1
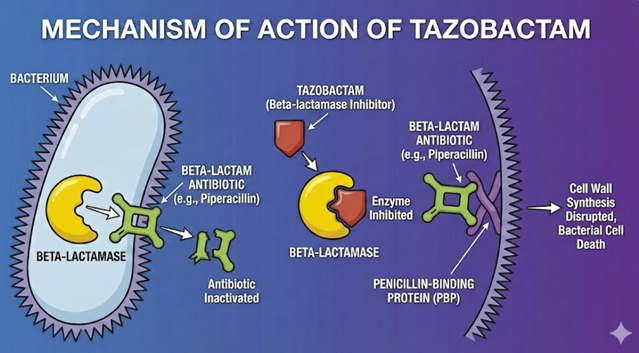
Tazobactam irreversibly inhibits many class A beta-lactamases by the same suicide-substrate mechanism as clavulanate and sulbactam.1,2
By binding and permanently disabling the enzyme, it protects partner beta-lactams from hydrolysis.
Tazobactam has negligible intrinsic antibacterial activity on its own.
It is currently used in combination with the ureidopenicillin piperacillin and with the cephalosporin ceftolozane.
While tazobactam is a potent inhibitor of most plasmid-mediated beta-lactamases (TEM, SHV, CTX-M ESBLs, etc.), it is not effective against some AmpC enzymes and not at all active against metallo-beta-lactamases.
Pharmacokinetics
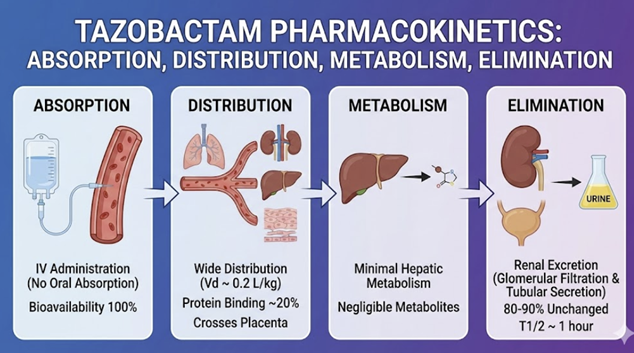 |
Tazobactam is only given parenterally (typically IV).
Tazobactam is administered intravenously, ensuring 100%
bioavailability.
In the combination piperacillin/tazobactam (Zosyn), the ratio is 8:1 (pip:tazo).8
After IV infusion, tazobactam’s pharmacokinetic profile is similar to that of piperacillin. Its half-life is about 0.7–1.0 hour.9
Tazobactam’s volume of distribution and clearance are roughly equivalent whether given alone or with piperacillin indicating minimal pharmacokinetic interaction between the two.9
About 56–64% of a tazobactam dose is excreted unchanged in urine in 24 hours.9
The remainder is likely eliminated as a single metabolite that appears to lack both pharmacological and antibacterial activities.10
Like other β-lactamase inhibitors, tazobactam is primarily renally cleared and accumulates in renal impairment (dose adjustment needed).11
Tazobactam also is a component of ceftolozane/tazobactam (ratio 2:1) – in that combination its kinetics remain linear and predictable, with similar reliance on renal elimination.
Ceftolozane/tazobactam combinations appear well tolerated by those patients with renal impairment.12
Therapeutic Uses: Piperacillin-tazobactam is considered a broad-spectrum antibiotic combination.
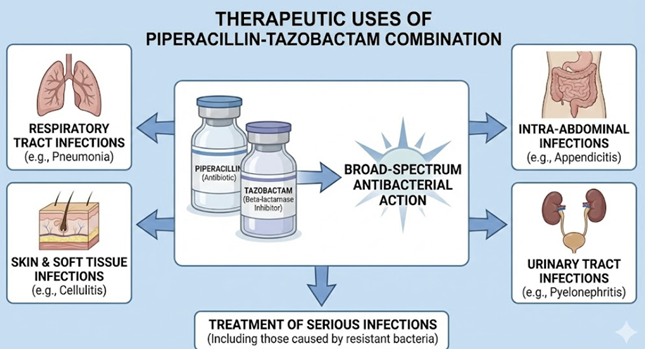 |
Intra-abdominal infections:
This category includes appendicitis with perforation, intra-abdominal abscesses, cholangitis.
Piperacillin-tazobactam covers gut Gram-negative rods which includes many beta-lactamase–producing E. coli, Klebsiella) and anaerobes (Bacteroides fragilis), in addition to enterococci, making it a one-drug regimen for polymicrobial abdominal infections.1
Complicated skin and soft tissue infections
This category includes diabetic foot infections, infected ulcers, where broad coverage of Gram-positives, Gram-negatives, and anaerobes is needed.13
Gynecologic infections
The indications include postpartum endometritis or pelvic infection, where mixed flora are involved.8
Serious hospital-acquired pneumonia
Piperacillin-tazobactam has activity against Pseudomonas aeruginosa (including many strains that produce beta-lactamases) and other nosocomial pathogens.
This combination is used for ventilator-associated pneumonia or severe aspiration pneumonia.
In combination with an aminoglycoside, it has been used in febrile neutropenia and other high-risk infections.1
Urinary tract infections (complicated)
Piperacillin-tazobactam covers (ESBL) Extended-Spectrum Beta-lactamase-producing organisms causing pyelonephritis or urosepsis when carbapenems are to be avoided.
August, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |