Medical Pharmacology Chapter 35 Antibacterial Drugs
Penicillins And Others
Beta-lactamase inhibitors
 |
 |
Mechanism of Action
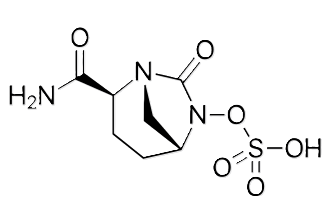
Avibactam representative substantial change in beta-lactamase inhibitor design.
This agent is a non-beta-lactamase inhibitor that exhibits a covalent, slowly reversible bond with beta-lactamases, with a deacylation rate of 0.045/min, i.e. about 4.5%/min1,5
The slowly reversible bond with beta-lactamases allows for sustained inhibition since the process regenerates intact inhibitor, allowing avibactam to reform its ring structure and bind again to another target lactamase.5,6
The reversible covalent mechanism add this to drug potency and enhances the spectrum of activity.
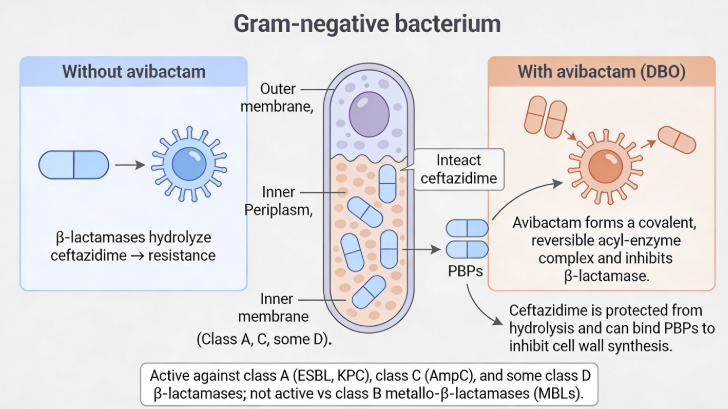
Avibactam is a potent inhibitor of Ambler Class A (including ESBLs [extended-spectrum beta-lactamases] and KPC [Klebsiella pneumoniae carbapenemase], Class C enzymes (AmpC),as well as some class D enzymes including OXA-48 carbapenemases.
Avibactam-mediated inhibition of both KPC and OXA-48 enhances its clinical importance targeting many carbapenem-resistance Enterobacterales (CRE, Carbapenem-resistant Enterobacterales).
![]() However, avibactam does not exhibit activity against
Class B metallo-beta-lactamases (MBLs).3,4
However, avibactam does not exhibit activity against
Class B metallo-beta-lactamases (MBLs).3,4
Pharmacokinetics
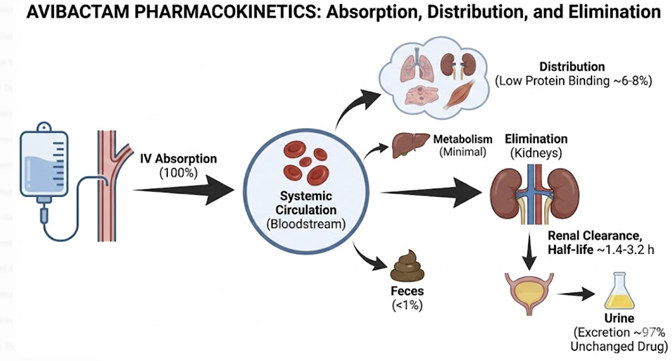 |
Absorption
Avibactam uses the intravenous route of administration (IV).7
Distribution
Avibactam exhibits very low plasma protein binding (5.7%-8.2%) with the volume of distribution of about 22 L.7, 8
Metabolism
Avibactam does not exhibit notable metabolism in humans.
Avibactam is eliminated from the body as the unchanged parent compound.6,7
Excretion
Elimination is almost exclusively via the kidneys.
Over 97% of an administered dose is recovered unchanged in the
urine.
Therapeutic Uses: ceftazidime-avibactam is appropriate for difficult Gram-negative infections. FDA-approved indications include:10,11
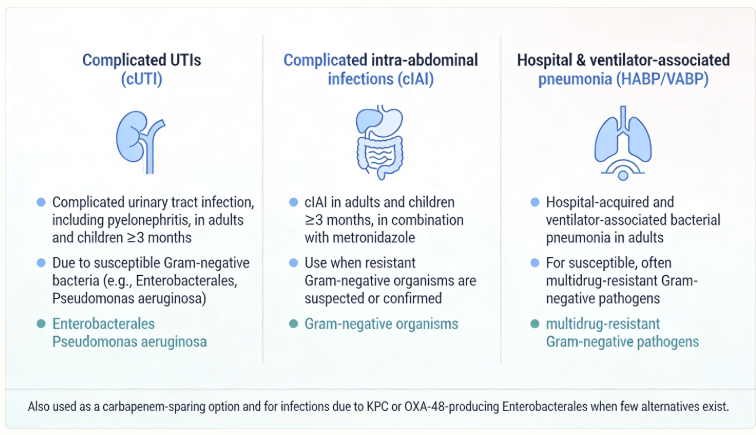 |
Complicated urinary tract infections (cUTIs), including pyelonephritis, due to susceptible Gram-negative bacteria (including many ESBL and some carbapenemase producers).
Complicated intra-abdominal infections (cIAIs) (used in combination with metronidazole for anaerobic coverage),
This combination is typically effective against resistant Enterobacteriaceae in intra-abdominal abscesses or infections.
Hospital-acquired and ventilator-associated pneumonia (HABP/VABP) due to Gram-negative rods, including P. aeruginosa and Enterobacterales that are not susceptible to other agents.11
August, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |