Medical Pharmacology Chapter 35 Antibacterial Drugs
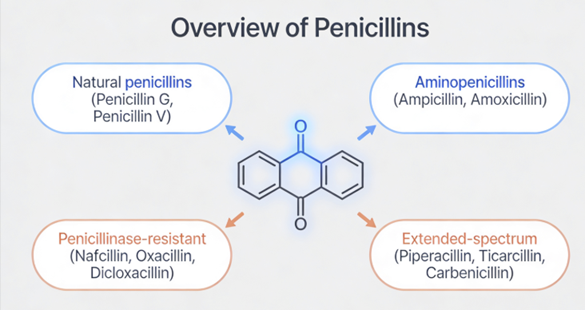 |
Penicillin overview
 |
|
 |
|
|
 |
|
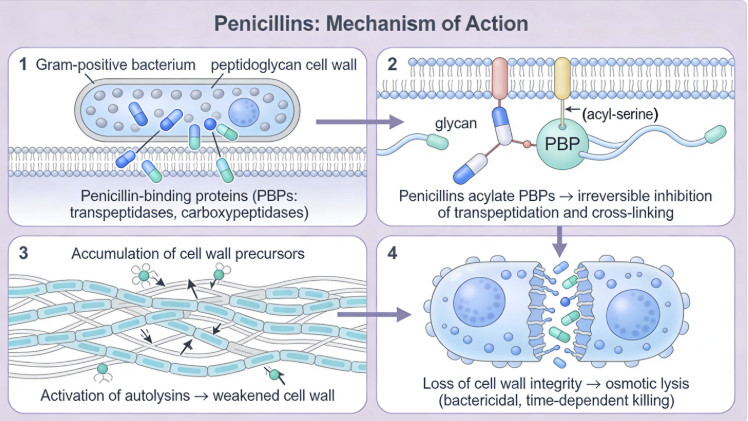
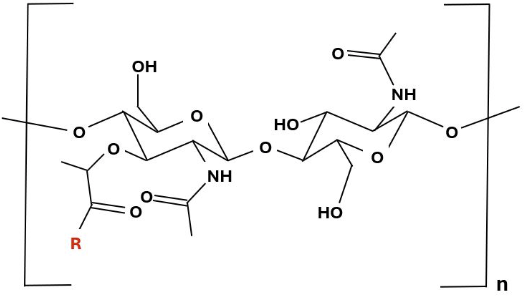 Peptidoglycan monomer in which the R group (red) represents a tetrapeptide.13 |
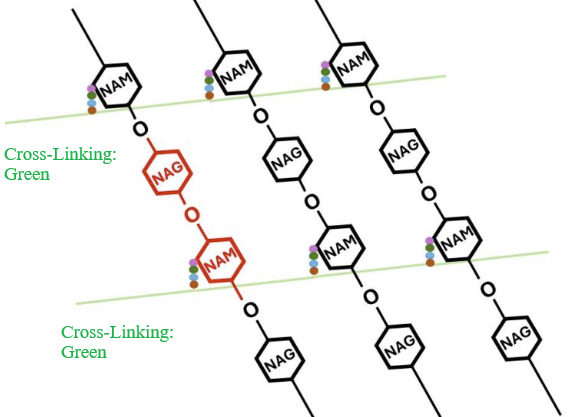 |
|
|
|
 |
|
 |
 |
 |
 |
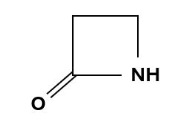
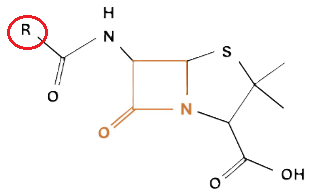
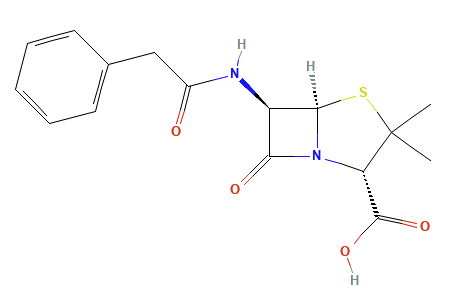
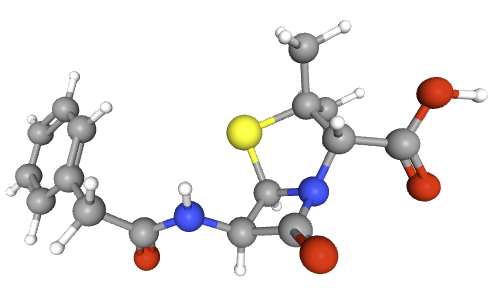
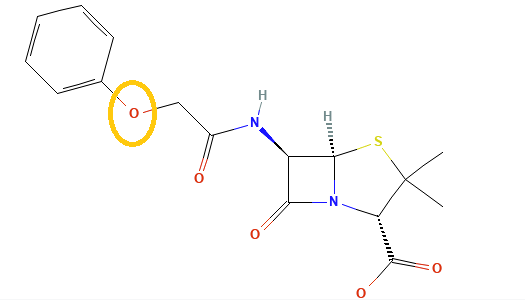
Penicillins, and all β-lactam antibiotics, are defined by their unique chemical architecture.
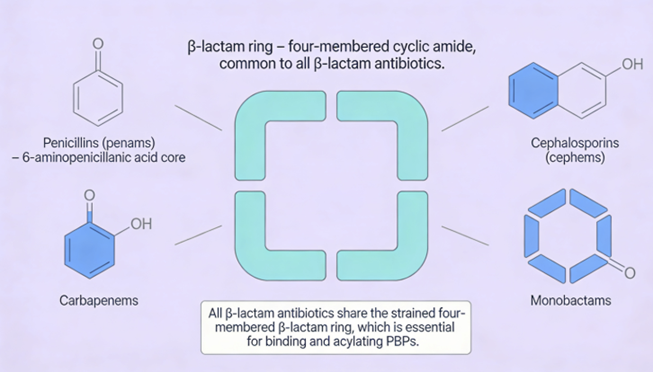 |
The core of the penicillin molecule is a bicyclic system
known as the penam nucleus.
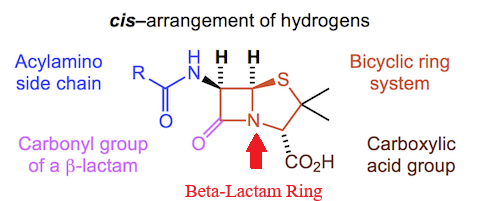 |
|
|
![]()
Chemically
definition(2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic
acid.
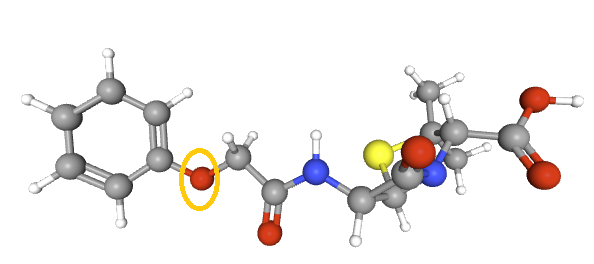
A defining feature is the benzyl (phenylacetyl) group that
constitutes its side chain.9
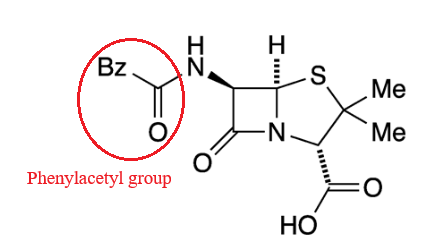 |
|
Penicillin G has also been known as benzylpenicillin or benzylpenicillinic acid.9
In its free acid form, penicillin G is a white
crystalline powder that is only sparingly soluble in
water and is chemically unstable, particularly in
aqueous solutions.
To overcome limitations with the free acid form,
penicillin G is formulated for example, as its
potassium salts, which are much more water-soluble
and stable, allowing for parenteral (intravenous or
intramuscular) administration.
To address this pharmacokinetic challenge, long-acting "depot" or "repository" formulations were developed.
These involve creating salts with large organic bases, such as procaine or benzathine, which are far less soluble in water.11,12
When injected intramuscularly, these salts form a depot from which the active penicillin G is slowly hydrolyzed and absorbed into the circulation over an extended period.
Procaine penicillin G provides therapeutic concentrations for approximately 24 hours11, while benzathine penicillin G can maintain low but effective levels for two to four weeks.12
August, 2025
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |