Medical Pharmacology Chapter 35 Antibacterial Drugs
Second Generation Cephalosporins: Cefoxitin
Cefoxitin Resistance Mechanism7
β-lactamase hydrolysis
Changed PBPs (for example,mecA/PBP2a) in MRSA
Reduced outer membrane permeability
AmpC β-lactamase–producing Enterobacterales (e.g., Enterobacter cloacae complex, Citrobacter freundii, Serratia marcescens) may show initial in-vitro susceptibility but can induce AmpC expression under cefoxitin exposure, leading to clinical failure.
EUCAST expert rules and IDSA AMR guidance emphasize the importance of recognizing AmpC phenotypes.9,10
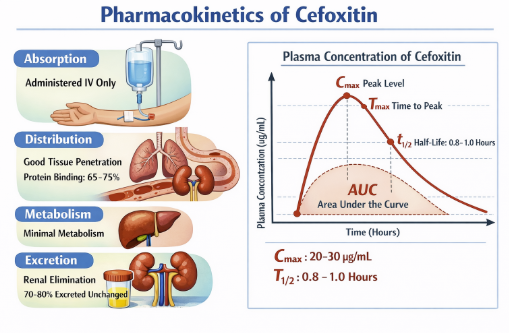 |
Basic Pharmacokinetic parameters11,12
Route of Administration: IV only (no oral bioavailability)11,14,15
Cefoxitin is available only for parenteral use (intravenous or intramuscular), and after IM administration its bioavailability is high, with ≥70–90% of the dose absorbed into the systemic circulation within several hours.
Following a typical IV dose, the serum concentration–time course is well described by a linear, two‑compartment open model, with a distribution phase followed by an elimination half‑life of approximately 45–80 minutes in adults with normal renal function.
Protein Binding
Protein binding is moderate (about 50–75%), and the apparent volume of distribution is low to moderate (around 0.3 L/kg), consistent with confinement largely to the extracellular fluid compartment.
Cefoxitin distributes widely into many tissues and fluids,
achieving therapeutic concentrations in peritoneal fluid,
pleural fluid, joint fluid, bile, and female genital tract
secretions, but penetration into cerebrospinal fluid is poor
in the absence of meningeal inflammation and is generally
insufficient for treatment of meningitis.14,15,16,17
Volume of distribution ≈ 10–15 L13
Serum half-life ~41–59 minutes in subjects with normal renal function
Total body clearance averages about 3–5 ml/min/kg in adults, and the elimination half‑life is significantly prolonged in renal impairment and by concomitant administration of probenecid, which competitively inhibits tubular secretion.11,17
In pregnancy, cefoxitin crosses the placenta with fetal:maternal ratios around 0.6 within less than an hour of maternal dosing, and it appears at low levels in breast milk, but available (though limited) human and animal data have not shown teratogenicity at therapeutic doses.16,18
Primarily renal clearance;
![]() accumulation with renal
impairment11, 14
accumulation with renal
impairment11, 14
Metabolism of cefoxitin is minimal, with roughly 2% or less of an administered dose undergoing biotransformation, so the parent compound accounts for nearly all antimicrobial activity.
Approximately 80–85% of the dose is excreted unchanged in the urine within 6 hours, by a combination of glomerular filtration and active tubular secretion; consequently, urinary concentrations are very high and the drug is efficiently cleared by the kidney.
 |
Overview
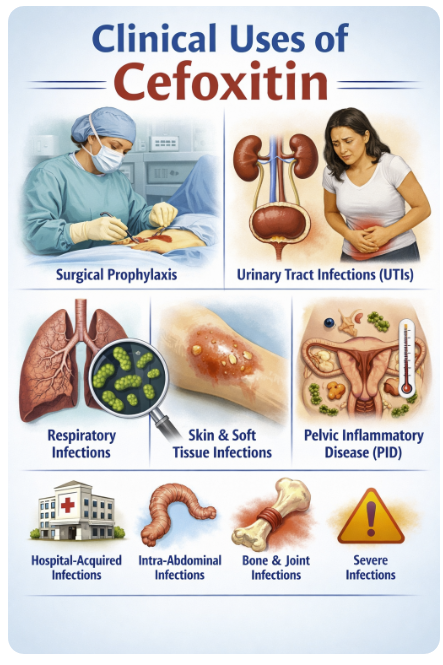
Cefoxitin is used primarily for infections where mixed aerobic–anaerobic flora from the gastrointestinal or genital tract are implicated, taking advantage of its anaerobic and Enterobacterales coverage in a single drug.19
With the availability of of third‑generation cephalosporins and β‑lactam/β‑lactamase inhibitor combinations, Cefoxitin use has narrowed but remains a rational choice in specific settings where cost, spectrum, and local resistance patterns so indicate.
Surgical Prophylaxis
Cefoxitin is a preferred agent for colorectal and abdominal surgery prophylaxis because it covers both aerobic Gram-negative rods and anaerobic flora.19
A classic use of cefoxitin is in complicated intra‑abdominal infections such as perforated appendicitis, diverticulitis with perforation or abscess, and postoperative peritonitis, where cefoxitin can cover typical Enterobacterales and anaerobes in community‑acquired disease of mild to moderate severity.16,21
Cefoxitin is also widely employed for obstetric and gynecologic infections, including:
Pelvic inflammatory disease (PID)
Postpartum endometritis, and
Septic abortion
U.S. CDC‑linked guidance has historically listed cefoxitin plus doxycycline as a first‑line regimen for in‑hospital management or single‑dose parenteral therapy of PID.
Cefoxitin is frequently used for surgical prophylaxis in procedures at high risk for contamination with colonic or vaginal flora, including colorectal surgery, some gynecologic operations (e.g., hysterectomy), and appendectomy.
Cefoxitin is usually administered as a single
pre‑incision dose (often with redosing in prolonged
operations), and prophylaxis is generally
discontinued within 24 hours to minimize selection
of resistant organisms and Clostridioides
difficile infection.22
Complicated Urinary Tract Infections and Pyelonephritis
Because of its high urinary excretion and potent activity against many Enterobacterales, cefoxitin can be used as an alternative agent for complicated urinary tract infections and pyelonephritis when local susceptibility patterns are favorable.
In orthopedic and soft‑tissue infections arising from bowel or perineal sources, such as sacral decubitus ulcers with osteomyelitis, cefoxitin can provide convenient single‑agent coverage for polymicrobial flora that include anaerobes and susceptible Gram‑negative bacilli.12,23
Pelvic Inflammatory Disease (PID)20
Cefoxitin is used in combination with doxycycline for inpatient treatment to cover N. gonorrhoeae and anaerobic vaginal pathogens.
![]() Cefoxitin: Adverse Reactions22,24,25,26
Cefoxitin: Adverse Reactions22,24,25,26
 |
Overview
![]() Adverse‑effect profile of cefoxitin is similar to that of
other parenteral cephalosporins, with most reactions being
mild and reversible but some serious toxicities requiring
vigilance in clinical practice.
Adverse‑effect profile of cefoxitin is similar to that of
other parenteral cephalosporins, with most reactions being
mild and reversible but some serious toxicities requiring
vigilance in clinical practice.
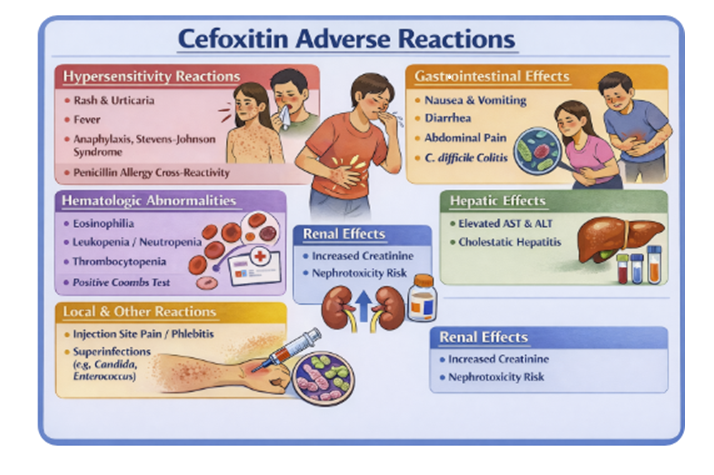
Common, generally self‑limited effects include local pain or inflammation at the injection site, mild gastrointestinal upset such as nausea and diarrhea, and transient laboratory abnormalities like eosinophilia or mild elevations in liver transaminases.
Hypersensitivity reactions
Simple morbilliform rash and pruritus
Urticaria
Angioedema
Serum sickness–like syndromes, and
Anaphylaxis (rarely) especially in patients with prior immediate‑type reactions to penicillins or other β‑lactams.
As with other broad‑spectrum antibiotics, cefoxitin can precipitate Clostridioides difficile–associated diarrhea and colitis, which may present during therapy or weeks to months after discontinuation, manifesting as severe, often bloody diarrhea, crampy abdominal pain, and systemic toxicity.21,25,27
Cefoxitin administration, like several second‑ and third‑generation cephalosporins, has been associated with positive Coombs tests and, rarely, immune hemolytic anemia, as well as thrombocytopenia, leukopenia, and eosinophilia, particularly with prolonged courses.
Nephrotoxicity is uncommon when cefoxitin is used alone but may occur, especially when combined with other nephrotoxic agents (aminoglycosides, high‑dose diuretics), and accumulation with neurotoxic manifestations (seizures, encephalopathy) can occur in severe renal impairment if dosage is not adjusted.21,25,27
Prolonged or repeated therapy may promote overgrowth of non‑susceptible organisms, including Candida (leading to oral thrush or vaginal candidiasis) and resistant Gram‑negative bacilli, necessitating clinical and microbiologic reassessment if there is failure to respond.
As with other cephalosporins, rare severe cutaneous adverse
reactions such as Stevens–Johnson syndrome and toxic
epidermal necrolysis have been reported, warranting
immediate discontinuation if blistering rash, mucosal
erosions, or systemic symptoms develop.25,26,27
January 2016
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation wCefoxitin. Johns Hopkins ABX Guide (last updated October 5, 2016). https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540097/all/Cefoxitinith practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |