Medical Pharmacology Chapter 35 Antibacterial Drugs
Cefuroxime: Audio Overview
 |
 |
 |
Introduction: Cefuroxime 2,3,4
![]() Cefuroxime
represents a pivotal agent in the second-generation
cephalosporin class, distinguished by its enhanced stability
against β-lactamase hydrolysis and expanded spectrum against
Gram-negative bacilli compared to first-generation
compounds.
Cefuroxime
represents a pivotal agent in the second-generation
cephalosporin class, distinguished by its enhanced stability
against β-lactamase hydrolysis and expanded spectrum against
Gram-negative bacilli compared to first-generation
compounds.
The drug exists in two primary formulations: cefuroxime sodium for parenteral administration and cefuroxime axetil, an acetoxyethyl ester prodrug that enables oral bioavailability through intestinal esterase hydrolysis.
This dual-route availability has positioned cefuroxime as a versatile option for transitioning patients from inpatient to outpatient therapy, particularly in community-acquired infections where antimicrobial stewardship principles favor step-down therapy to narrow-spectrum agents.
Mechanism of Action: Cefuroxime.2,3,5,6
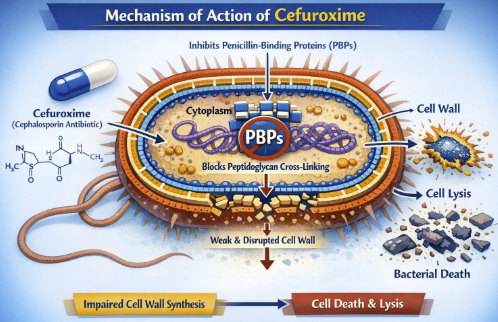 |
![]() The
bactericidal activity of cefuroxime originates from its
high-affinity binding to penicillin-binding proteins (PBPs),
essential enzymes responsible for the final cross-linking of
peptidoglycan units during bacterial cell wall synthesis.
The
bactericidal activity of cefuroxime originates from its
high-affinity binding to penicillin-binding proteins (PBPs),
essential enzymes responsible for the final cross-linking of
peptidoglycan units during bacterial cell wall synthesis.
Cefuroxime demonstrates preferential affinity for PBP3, while maintaining inhibitory activity against PBP1a and PBP1b.
This binding occurs through the β-lactam ring structure, which sterically inhibits the transpeptidation reaction required for cell wall integrity.
Resulting inhibition prevents formation of the rigid peptidoglycan network that protects bacteria from osmotic lysis, leading to cell death through autolytic enzyme activation.
Cefuroxime is resistant to hydrolysis by many β-lactamases produced by Gram-negative organisms, a property that distinguishes it from earlier cephalosporins and penicillins.
![]() This resistance to enzymatic degradation is
particularly evident against TEM-type β-lactamases
in Haemophilus influenzae and
Neisseria gonorrhoeae, though it remains
vulnerable to extended-spectrum β-lactamases
(ESBLs) and AmpC cephalosporinases.
This resistance to enzymatic degradation is
particularly evident against TEM-type β-lactamases
in Haemophilus influenzae and
Neisseria gonorrhoeae, though it remains
vulnerable to extended-spectrum β-lactamases
(ESBLs) and AmpC cephalosporinases.
However, cefuroxime does not bind altered PBPs in Methicillin-Resistant Staphylococcus Aureus (MRSA) or penicillin-resistant Streptococcus pneumoniae, so these strains are inherently resistant.2
The failure to bind PBP2a (encoded by mecA in MRSA) represents the basis for cefuroxime’s lack of efficacy against MRSA.2
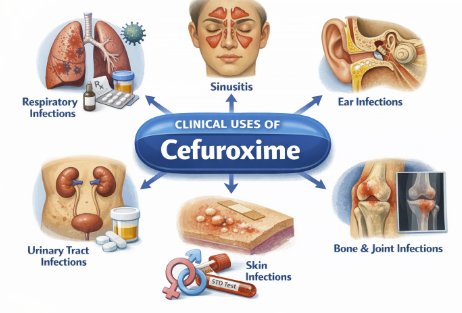
Antimicrobial Spectrum and Resistance2,7,8,9
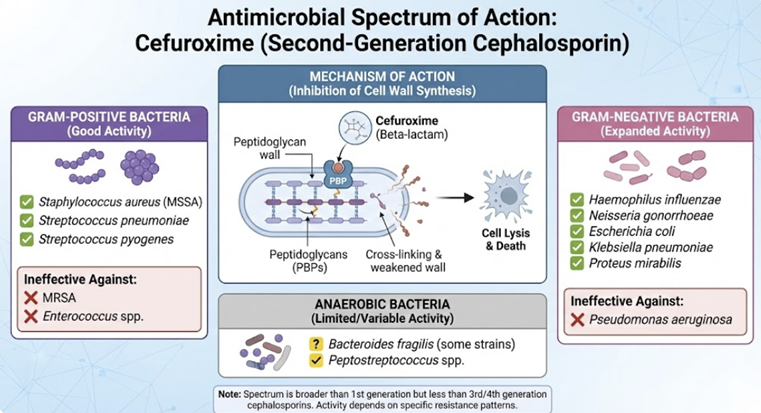 |
Cefuroxime has a broad antibacterial spectrum encompassing many gram-positive and gram-negative organisms.
Cefuroxime is active against Staphylococcus aureus (methicillin-susceptible strains), Streptococcus pyogenes, and S. pneumoniae, although activity against enterococci and MRSA is poor.
Cefuroxime covers many common gram-negative respiratory pathogens including Haemophilus influenzae (even ampicillin-resistant strains via β-lactamase production), Moraxella catarrhalis, and Neisseria gonorrhoeae (including penicillinase-producing strains).
This agent is also generally effective against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Salmonella, Shigella, Providencia, and others.
Cefuroxime exhibits good activity in vitro against many Enterobacter and Citrobacter species, but some strains of these organisms can inducibly express cephalosporinases that confer resistance.
![]() Anaerobic coverage of cefuroxime is limited with
some activity against some anaerobes (like
Peptostreptococcus, Clostridium (non-C.
difficile species), Fusobacterium),
but it is ineffective against Bacteroides
fragilis and Clostridioides difficile.
Anaerobic coverage of cefuroxime is limited with
some activity against some anaerobes (like
Peptostreptococcus, Clostridium (non-C.
difficile species), Fusobacterium),
but it is ineffective against Bacteroides
fragilis and Clostridioides difficile.
Cefuroxime is usually ineffective against Pseudomonas aeruginosa, certain Proteus (e.g. P. vulgaris), Campylobacter, Acinetobacter, and most Serratia, which are intrinsically resistant.
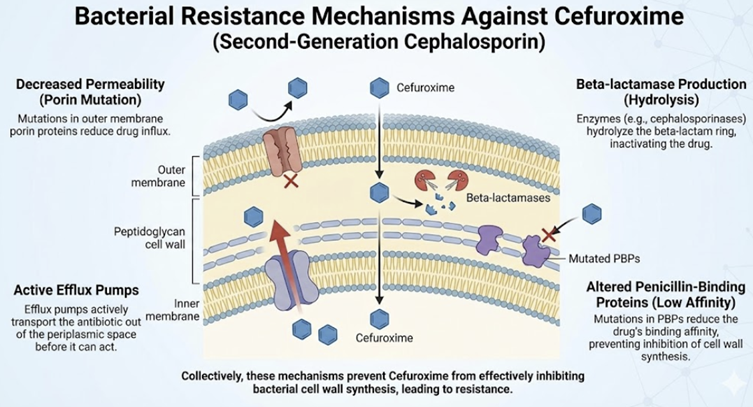
Main mechanisms of resistance to cefuroxime include bacterial production of β-lactamase enzymes that hydrolyze the drug (though cefuroxime is stable against many common plasmid β-lactamases), modifications of PBPs (e.g. PBP2a in MRSA), and reduced permeability of gram-negative outer membranes hindering drug entry.
 |
|
Pathogen |
Cephalexin (1st Gen) |
Cefuroxime (2nd Gen) |
Clinical Importance |
|
Escherichia coli |
Active |
Active |
Both are effective for uncomplicated UTIs; however, resistance is growing for both (FDA Zinacef Labeling, 2024)11 |
|
Klebsiella pneumoniae |
Variable |
Active |
Cefuroxime provides more reliable coverage for community-acquired Klebsiella infections.12 |
|
Proteus mirabilis |
Active |
Active |
Common urinary pathogen covered by both generations. |
|
Haemophilus influenzae |
Weak/Inactive |
Highly Active |
Cefuroxime is stable against many β-lactamases that render 1st Gen agents useless10 |
|
Moraxella catarrhalis |
Inactive |
Highly active |
Essential for treating acute otitis media and exacerbations of COPD.2 |
|
Neisseria gonorrhea |
Inactive |
Active |
Historically used for gonorrhea, though third-generation agents are now the gold standard. |
|
Enterobacter species |
Resistant |
Variable/Weak |
Generally, neither should be used for Enterobacter due to potential AmpC induction. |
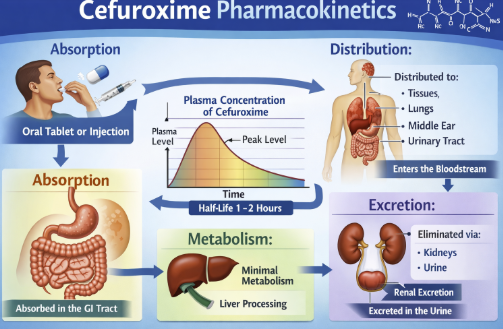 |
Absorption and Bioavailability2,3
The oral form, cefuroxime axetil, is an acetoxyethyl ester prodrug that is poorly absorbed as the parent cefuroxime but becomes readily absorbed after conversion in the gut.
After oral administration, cefuroxime axetil is hydrolyzed by esterases in the intestinal mucosa to release active cefuroxime, which enters the circulation.
Absorption is significantly enhanced by food – the bioavailability of oral cefuroxime axetil is about 37% on an empty stomach, rising to approximately 52% when taken with food.
In adults, peak serum concentrations are reached about 2–3 hours after an oral dose, whereas in young children peak levels occur slightly later (around 3–4 hours).
Intramuscular or intravenous administration of cefuroxime bypasses this absorption step, and with IV infusion peak levels are achieved within minutes.
Parenteral administration achieves rapid therapeutic concentrations, with intravenous injection producing peak serum levels within 2-3 minutes and intramuscular injection reaching maximum concentration in 2-3 hours.
Distribution and Protein Binding2,8,13
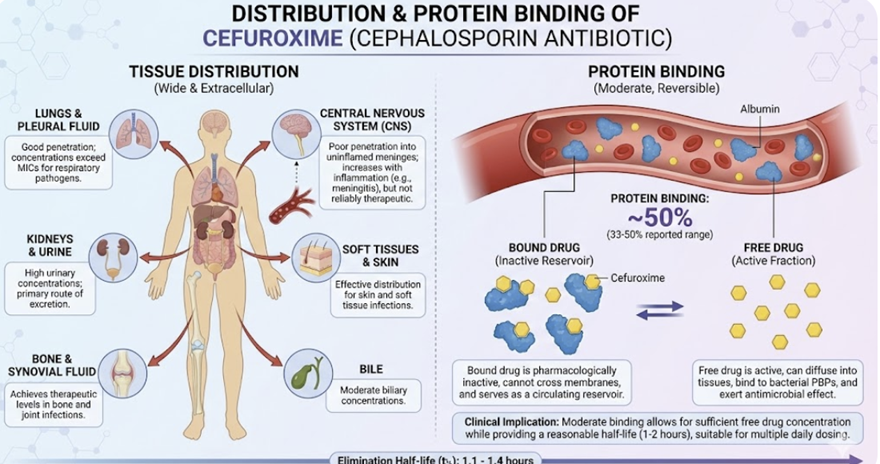 |
Distribution
Cefuroxime has a moderate volume of distribution (~0.25–0.3 L/kg) and is roughly 33–50% bound to plasma proteins, distributing reasonably well into various tissues and body fluids. However, this agent exhibits less tissue penetration compared to the newer cephalosporins.
Therapeutically effective concentrations are attained in the respiratory tract (e.g. lung tissue, bronchial mucosa), ENT tissues (e.g. sinuses, tonsils, and middle ear effusions), and even the aqueous humor of the eye.
Cefuroxime can penetrate into bone, which is useful for certain orthopedic or odontogenic infections.
Cefuroxime also crosses the inflamed meninges to reach the cerebrospinal fluid (CSF) at therapeutic levels, allowing it to be used for susceptible bacterial meningitis (although third-generation cephalosporins are more commonly preferred for CNS infections due to superior CSF penetration).
Protein Binding
Cefuroxime exhibits moderate protein binding, with approximately 33-50% of serum cefuroxime bound to albumin.
This binding is clinically advantageous, as it provides a reservoir of drug while maintaining sufficient free fraction for antimicrobial activity.
Metabolism and Elimination2,13,14,16
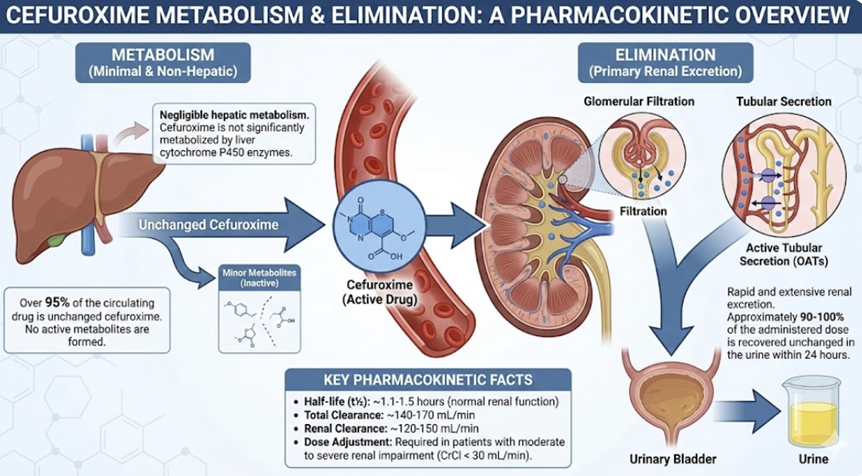 |
![]() Cefuroxime
undergoes minimal hepatic metabolism; the primary metabolic
transformation involves cleavage of the axetil moiety in the
intestinal mucosa to release active cefuroxime.
Cefuroxime
undergoes minimal hepatic metabolism; the primary metabolic
transformation involves cleavage of the axetil moiety in the
intestinal mucosa to release active cefuroxime.
Unchanged drug is predominantly eliminated via renal excretion, with approximately 50% of the administered dose recovered in urine within 12 hours.
Renal tubular secretion accounts for 43-54% of total renal clearance, as demonstrated by probenecid-induced reductions in clearance of approximately 40%.
Eimination drug half-life is about 60-90 minutes in adults with normal renal function, extending to 1.4-1.9 hours in pediatric patients.15
In the presence of renal impairment, dose adjustments become necessary when creatinine clearance falls below 50 mL/min/1.73 mē.
![]() Special Populations15,16
Special Populations15,16
Patients with impaired renal function require systematic dose modifications to prevent accumulation and toxicity.
When creatinine clearance ranges from 30-49 mL/min, dosing intervals should extend to every 12 hours; at 10-29 mL/min, every 24 hours; and below 10 mL/min, every 48 hours.
For patients undergoing hemodialysis, 750 mg twice daily provides adequate coverage, while continuous arteriovenous hemofiltration may require 750 mg twice daily for high-flux systems.
Pediatric pharmacokinetics show similar elimination characteristics to adults beyond three weeks of age, though neonates exhibit prolonged half-lives due to immature renal function.
Cefuroxime: Pharmacodynamics2,17
Similar to other β-lactams, cefuroxime exhibits time-dependent killing (efficacy is linked to the duration plasma concentrations exceed the minimum inhibitory concentration of the pathogen).
Cefuroxime is generally bactericidal against susceptible bacteria and has a post-antibiotic effect of short duration.
Combining cefuroxime with a bacteriostatic agent is usually not problematic, but in severe infections cefuroxime may be used with an aminoglycoside for synergistic effect (especially to broaden coverage).
Probenecid can increase cefuroxime levels by blocking its tubular secretion .
This interaction has been used therapeutically to prolong β-lactam
levels (but such combination must be accompanied by
increased concern about drug exposure and toxicity.
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |