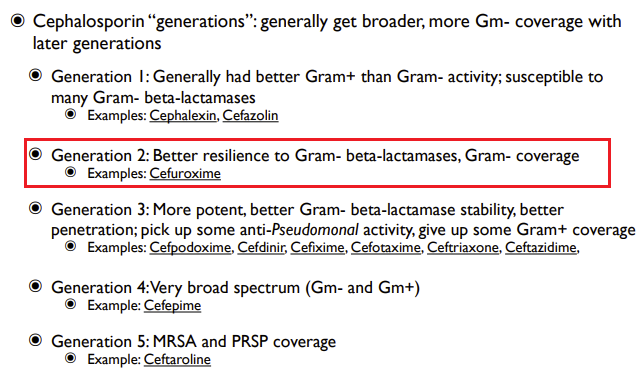
Medical Pharmacology Chapter 35 Antibacterial Drugs
Second Generation Cephalosporins: Cefuroxime
| Parenteral Administration |
 |
|
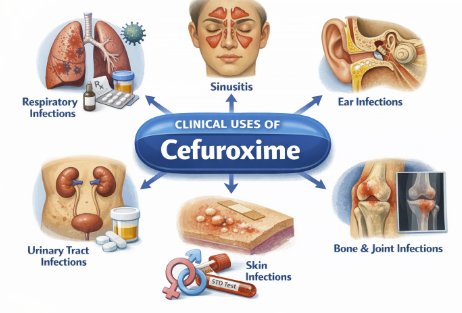
Cefuroxime: Therapeutic Uses (Clinical Applications) (Parenteral Administration)
 |
|
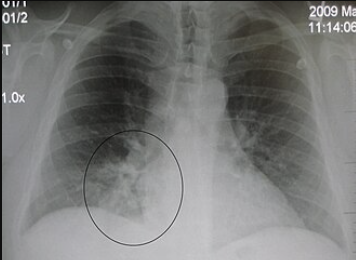
Lower Respiratory Tract Infections (Parenteral Administration): Cefuroxime (IV/IM) is indicated for community-acquired pneumonia and other lower respiratory infections caused by susceptible bacteria. (Parenteral Administration)
 |
|
Cefuroxime is effective against S. pneumoniae, H. influenzae (including β-lactamase-producing strains), Staphylococcus aureus (MSSA), Streptococcus pyogenes, and Klebsiella in the lungs.2,5,6
The Infectious Diseases Society of America (IDSA) and American Thoracic Society have endorsed cefuroxime as an option for hospitalized community-acquired pneumonia.
In practice, IV cefuroxime for pneumonia is often paired with a macrolide to cover atypical pathogens, or a respiratory fluoroquinolone might be used instead; however, cefuroxime is a reasonable β-lactam choice for many typical pneumonia cases.
Cefuroxime is also used for bacterial bronchitis or pneumonia in patients with chronic lung disease when those above organisms are suspected.2,5
Upper Respiratory Tract Infections (Parenteral Administration): Although first-line therapy for many ENT infections is often amoxicillin or amoxicillin-clavulanate, cefuroxime is indicated as an alternative in certain situations.2,7,8
Acute bacterial maxillary sinusitis can be treated with cefuroxime in adults and adolescents (≥13 years old) when due to susceptible H. influenzae or S. pneumoniae.
Acute otitis media (AOM) is also an indication: cefuroxime axetil is effective for middle ear infections caused by H. influenzae, S. pneumoniae, S. pyogenes, or M. catarrhalis, including β-lactamase–producing strains.
In pediatric practice, cefuroxime is often used for recurrent or persistent Acute Otitis Media (AOM), especially if the pathogen might be amoxicillin-resistant.
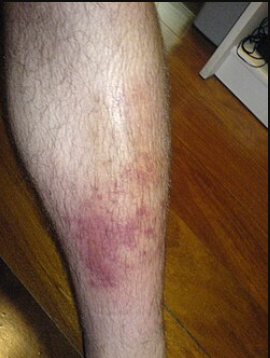
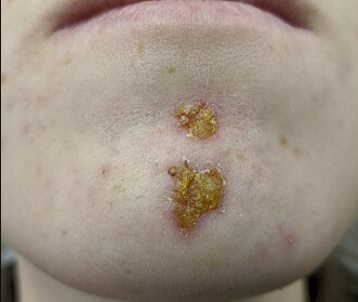
Skin and Soft Tissue Infections (Parenteral Administration)2,9,10
Cefuroxime is indicated for skin and skin-structure infections (SSTIs) such as cellulitis, wound infections, or impetigo, caused by susceptible strains of Staphylococcus aureus (MSSA, β-lactamase positive or negative) and Streptococcus pyogenes.
 |
|
 |
|
Cefuroxime covers some Gram-negative causes of skin infections (e.g. certain E. coli or Klebsiella in wound infections).
Uncomplicated skin and soft tissue infections (SSTIs) in adults and adolescents (≥13) can be treated with oral cefuroxime axetil, whereas more serious SSTIs may require IV cefuroxime.
Oral cefuroxime serves as a useful step-down therapy after IV antibiotics for skin infections, or as an alternative for patients who cannot take penicillins.
Cefuroxime is also effective for impetigo (a superficial skin infection) in children.
An oral suspension course can treat impetigo caused by Staphylococcus or Streptococcus.2
Urinary Tract Infections (Parenteral Administration)2,11,12
Cefuroxime is indicated for uncomplicated UTIs (e.g. cystitis) caused by susceptible organisms, particularly Escherichia coli and Klebsiella pneumoniae.
While first-line treatments for simple UTIs are often trimethoprim-sulfamethoxazole or nitrofurantoin, cefuroxime is an option especially if the pathogen is known to be susceptible.
Cefuroxime achieves high concentrations in urine due to renal excretion.
Parenteral cefuroxime can also treat pyelonephritis (kidney infections) caused by these bacteria, though in severe cases a broader-spectrum IV antibiotic might be chosen.13,14
Septicemia (Parenteral Administration)2,15
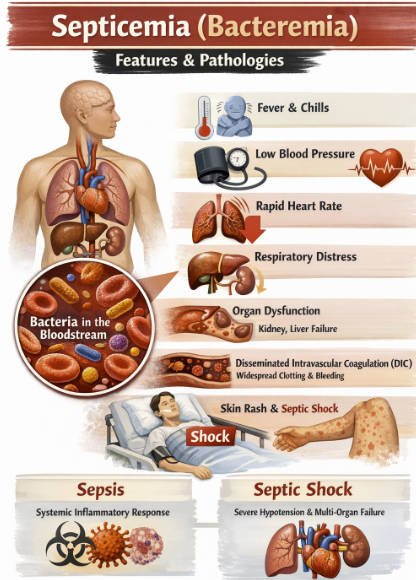 |
IV cefuroxime is approved for treating septicemia (bacteremia) caused by susceptible organisms.
Cefuroxime for this indication includes sepsis due to:
Staphylococcus aureus (MSSA)
Streptococcus pneumoniae
H. influenzae
E. coli
Klebsiella and others if the isolate is sensitive to cefuroxime.
![]() Management
of sepsis depends on the source of infection and local
resistance patterns.
Management
of sepsis depends on the source of infection and local
resistance patterns.
For example, cefuroxime would be appropriate for sepsis arising from a community-acquired pneumonia, for example, but would not be adequate for Pseudomonas sepsis.
For life-threatening infections like sepsis or endocarditis, third-generation cephalosporins or other agents might be more frequently used unless cefuroxime’s spectrum is a perfect match for the identified pathogen.
Meningitis (Parenteral Administration)2,16,17
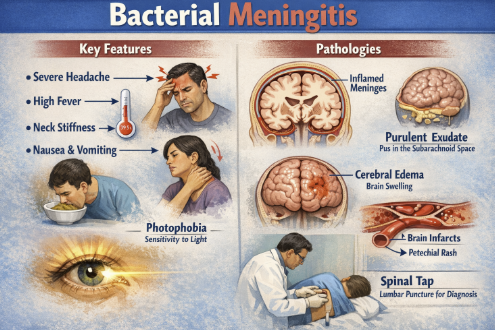 |
Cefuroxime is one of the few second-generation cephalosporins that achieves therapeutic levels in cerebrospinal fluid when meninges are inflamed.
Cefuroxime administration is indicated for bacterial meningitis caused by susceptible strains of Neisseria meningitidis, Streptococcus pneumoniae, Haemophilus influenzae (including ampicillin-resistant strains), or Staphylococcus aureus (MSSA).2
Prior to the widespread use of third-generation cephalosporins, cefuroxime was used in meningitis treatment.
H. influenzae meningitis and meningococcal meningitis can be treated with IV cefuroxime.
Current guidelines generally favor ceftriaxone or cefotaxime (third-gen cephalosporins) for empiric meningitis therapy because of their enhanced potency and CNS penetration.
Cefuroxime may still be employed in penicillin-allergic patients (without anaphylaxis) or in targeted therapy when the organism is known to be cefuroxime-susceptible and third-generation cephalosporins are contraindicated.
Cefuroxime is not effective against Listeria monocytogenes, an important cause of meningitis in neonates and the elderly, so it would never be monotherapy in those groups.2
Gonococcal Infections (Parenteral Administration)2,18,19
Cefuroxime has activity against Neisseria gonorrhoeae, including penicillinase-producing gonococcal strains.
Cefuroxime was historically used for uncomplicated gonorrhea.
The FDA-approved regimen for gonorrhea (per older labeling) was a single IM dose of 1.5 g cefuroxime (usually split into two 750 mg injections given simultaneously at two sites), combined with oral probenecid to prolong levels.
Given rising resistance, ceftriaxone has become the recommended treatment for gonorrhea worldwide, and cefuroxime is no longer a preferred agent.
CDC guidelines do not list cefuroxime as first-line for gonorrhea today.
In disseminated gonococcal infections (e.g. gonococcal arthritis), ceftriaxone is again preferred over cefuroxime.
Bone and Joint Infections (Parenteral Administration)2,3,20
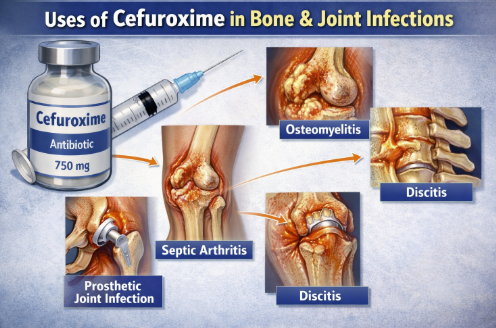 |
Cefuroxime may be appropriate for infections of bones and joints caused by susceptible organisms, primarily MSSA.
Cefuroxime could be used for septic arthritis or osteomyelitis due to MSSA, as an alternative to oxacillin/cefazolin.
Usually first-generation cephalosporins (like cefazolin) or antistaphylococcal penicillins are often favored for MSSA bone/joint infections.
Cefuroxime administration is associated with good bone penetration making it useful if those first-line agents cannot be used (e.g. due to mild penicillin allergy).
Cefuroxime may also be chosen for polymicrobial diabetic foot infections in combination with anaerobic coverage, although for broad coverage other regimens are usually selected.21
February, 2026
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |