Medical Pharmacology Chapter 35 Antibacterial Drugs
Second Generation Cephalosporins: Cefuroxime
Cefuroxime: Adverse Reactions2,3
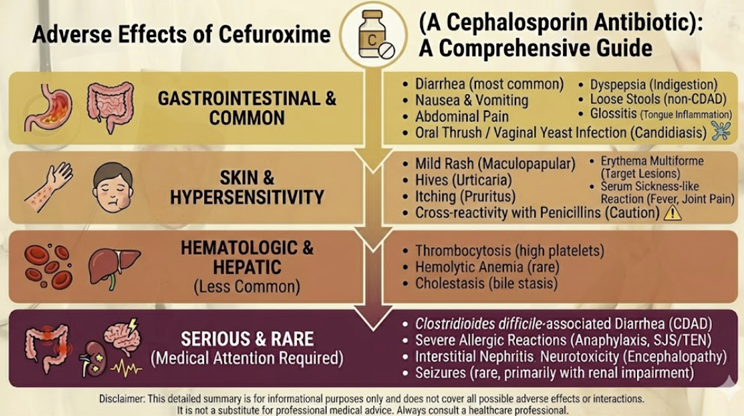 |
Gastrointestinal2,12
The most frequently reported adverse reactions to cefuroxime involve the gastrointestinal tract.
Nausea, vomiting, diarrhea, and abdominal pain occur in a dose-dependent manner, with oral formulations generating more GI upset than parenteral administration.
In clinical trials examining oral cefuroxime axetil for cystitis, 15.6% of patients reported adverse events, with diarrhea/loose motions being the most frequent gastrointestinal manifestation.
These reactions are typically mild and transient, often resolving spontaneously during continued therapy.
Hypersensitivity Reactions and IgE-Mediated Anaphylaxis5,6,7
Although cefuroxime exhibits a overall favorable safety profile, IgE-mediated hypersensitivity reactions ranging from benign cutaneous reactions to life-threatening anaphylaxis can occur.
Anaphylaxis to cefuroxime is rare but well-documented, manifesting within seconds to minutes of drug exposure with symptoms including throat itching, tongue and angioedema, generalized urticaria, dyspnea, hypotension, and loss of consciousness.
Serum tryptase measurement provides objective documentation, with levels typically elevated above 13 µg/L during acute anaphylactic events, peaking within 1-2 hours and normalizing by 24 hours.
Anaphylaxis can occur even in patients with prior cefuroxime exposures that were tolerated without adverse events, indicating that sensitization may develop over time.
Diagnostic evaluation of cefuroxime anaphylaxis employs skin prick testing and specific IgE assays, though false-negative results occur and sensitivity/specificity remain imperfect.
Cross-reactivity to penicillins in patients developing cefuroxime anaphylaxis is minimal, as demonstrated by negative penicillin skin tests even in patients with documented cefuroxime-specific IgE.6,7
Cross-Reactivity with Penicillins and Other β-Lactams8,9,10,11
![]() Contemporary
meta-analyses of patients with reported penicillin allergy
demonstrate that the risk of clinically significant
cross-reactivity with second and third-generation cephalosporins
such as cefuroxime is less than 2% (specifically 0.7-2.0%).
Contemporary
meta-analyses of patients with reported penicillin allergy
demonstrate that the risk of clinically significant
cross-reactivity with second and third-generation cephalosporins
such as cefuroxime is less than 2% (specifically 0.7-2.0%).
This low cross-reactivity rate reflects fundamental differences in the R1 and R3 side chains of cefuroxime compared to penicillin and amoxicillin, with the dissimilar side chains minimizing epitope recognition by penicillin-specific IgE antibodies.
Cefuroxime is considered a safe alternative for most patients with documented true penicillin allergy, provided that severe immediate-type reactions such as anaphylaxis, Stevens-Johnson syndrome, or toxic epidermal necrolysis have not previously occurred.
In patients with penicillin allergy reporting non-severe reactions (delayed rashes, oral tolerance), preoperative penicillin allergy testing has demonstrated that over 95% of patients test negative and can safely receive cephalosporin prophylaxis.
In patients with pre-existing beta-lactam sensitization, cefuroxime maintains a 6.3% hypersensitivity rate in sensitized populations, and 4.2% in those specifically sensitized to penicillin alone.
Even in penicillin-allergic patients, cefuroxime offers substantially reduced allergic risk compared to other beta-lactams.13
Severe Cutaneous Adverse Reactions: Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
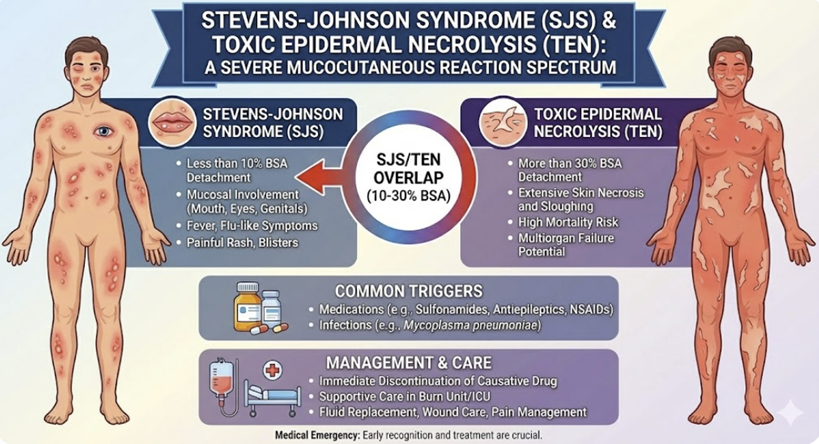 |
![]() Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
(TEN) represent rare but catastrophic complications of
cefuroxime therapy, with mortality rates for TEN reaching
30-50%.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
(TEN) represent rare but catastrophic complications of
cefuroxime therapy, with mortality rates for TEN reaching
30-50%.
These severe cutaneous adverse reactions typically present with an initial prodromal period characterized by fever and constitutional symptoms, followed by the appearance of widespread blistering, erythema, and eventual epidermal detachment.
The latency period between cefuroxime exposure and SJS/TEN development varies from days to weeks, necessitating vigilant patient monitoring throughout therapy and in the weeks following treatment completion.
The European Medicines Agency has issued formal warnings emphasizing that any sign of serious cutaneous reaction mandates immediate drug discontinuation and emergency evaluation.14
Hematologic Manifestations2,8,14
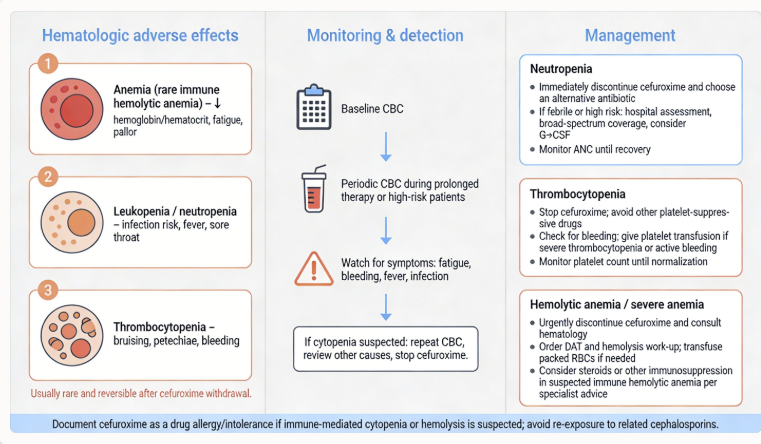 |
Reports of cefuroxime-associated hematologic abnormalities include decreased hemoglobin or hematocrit, eosinophilia, and prolonged prothrombin time.
Mechanisms underlying these effects vary; eosinophilia likely represents a hypersensitivity phenomenon, while prothrombin time prolongation may reflect intestinal dysbiosis with reduced bacterial synthesis of vitamin K-dependent factors.
In patients with risk factors for coagulopathy (poor nutritional status, renal disease, prolonged antibiotic therapy, or concomitant anticoagulation), monitoring of prothrombin time or INR is advisable, with vitamin K supplementation administered when warranted.
Clostridioides difficile-Associated Disease
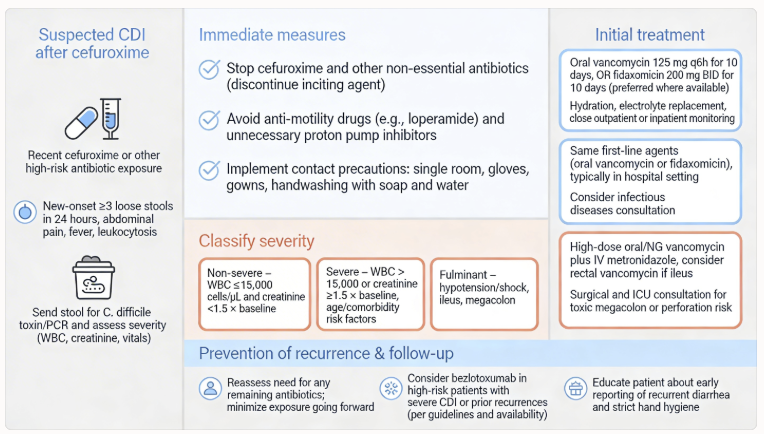 |
Cefuroxime use disrupts normal colonic flora, increasing susceptibility to Clostridioides difficile infection.
Prolonged or repeated courses substantially elevate risk.
C. difficile colitis presents with watery diarrhea, abdominal cramping, and systemic toxicity, potentially progressing to toxic megacolon and septic shock in severe cases.
Management requires prompt discontinuation of cefuroxime, assessment of disease severity, and initiation of anti-C. difficile therapy with oral vancomycin or fidaxomicin.
Neurologic Adverse Effects
A post-marketing surveillance study identified a statistically significant elevated risk of delirium in hospitalized patients receiving several antibiotics including cefuroxime.
While the exact mechanism remains unclear, high doses in patients with renal impairment create potential for neurotoxicity.
![]() Cefuroxime overdosage can induce cerebral irritation potentially
resulting in seizures, particularly in patients with renal
insufficiency or prior seizure history.
Cefuroxime overdosage can induce cerebral irritation potentially
resulting in seizures, particularly in patients with renal
insufficiency or prior seizure history.
Patients requiring high doses should undergo appropriate dose reductions for renal impairment, and neurologic status should be monitored vigilantly.
February, 2026
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |