|
|
|
Medical Pharmacology Chapter 33-34: Anticancer Drugs
Antimetabolites
Purine Analogues:
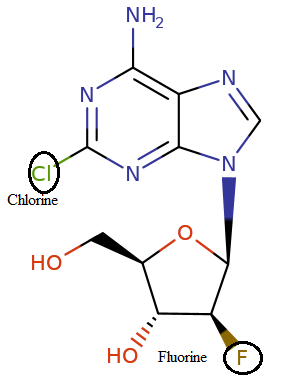
Clofarabine (Clolar)
 |
 |
Clofarabine is classified as a second-generation purine nucleoside analog which must be activated by sequential phosphorylation steps intracellularly.9
![]() The cytotoxic
active form, the triphosphate metabolite, is an enzyme inhibitor of both DNA
polymerase and ribonucleotide reductase.
The cytotoxic
active form, the triphosphate metabolite, is an enzyme inhibitor of both DNA
polymerase and ribonucleotide reductase.
A consequence of inhibition of these enzymes is both DNA repair dysfunction and inhibition of DNA and RNA synthesis .
Clofarabine also is disruptive to mitochondrial function in membrane integrity.
This effect results in release of pre-apoptotic factors leading to programmed cell death.
Pre-apoptotic factors include both cytochrome C and apoptotic-inducing molecules.9
![]() Clofarabine (Clolar)
may be used for treating children who suffer from relapsed or refractory
acute lymphoblastic leukemia (ALL), after the use of at least two previous
treatment protocols.1,8
Clofarabine (Clolar)
may be used for treating children who suffer from relapsed or refractory
acute lymphoblastic leukemia (ALL), after the use of at least two previous
treatment protocols.1,8
In such circumstances clofarabine administration results in complete remission in about 25% of this subgroup of patients.1
In addition clofarabine exibits anti-cancer activity in pediatric and adult acute myelogenous leukemia (AML) and in myelodysplasia.
|
|
|
|
Clofarabine administration results in increasing intracellular cytosine arabinoside, thus enhancing ara-C cytotoxicity.1
Both uptake and metabolic activation of clofarabine in tumor cells proceed along the same course as cladribine and other purine nucleosides.
In the activated triphosphate form, clofarabine triphosphate exhibits a relatively long intracellular t1/2 (halftime) of about one day.
Following incorporation into DNA, clofarabine triphosphate results in DNA synthesis termination and activation of apoptosis (programmed cell death).1
Clofarabine is also inhibitor of RNR (ribonucleotide reductase).1
Clofarabine (2-chloro-2'-arabinosyladenenine) incorporates a 2-chloro substituent of cladribine which confers glycosylase resistance.1
Also incorporated is a 2'-fluoro-arabinosyl substitution which both improves drug uptake and phosphorylation (thus enhancing activation by deoxycytidine kinase) as well as enhancing drug stability.1
Clofarabine is, following cell uptake, converted to the 5'-triphosphate metabolite by means of a process comparable to that seen with cladribine and fludarabine activation.8
However, clofarabine appears to be a better substrate compared to fludarabine or cladribine for deoxycytidine kinase.8
The activated triphosphate form inhibits both DNA synthesis and repair.
![]() Following DNA
incorporation:
Following DNA
incorporation:
Clofarabine nucleotide inhibits ribonucleotide reductase (RNR),
Inhibits DNA polymerases and
Depletes intracellular deoxynucleotides.
Elongation of DNA strands is also inhibited.
Binding to the active site of RNR both decreases dATP levels while promoting clofarabine triphosphate incorporation into DNA.
Clofarabine administration promotes apoptosis by causing mitochondrial damage.8
Absorption, Distribution, Biotransformation, Excretion
In children, clofarabine is administered by IV infusion (2-hour infusion per day for 5 days).1
The principal elimination half-time (t1/2) is about 6.5 hours.1
About 50% of the administered dose is excreted in the urine unchanged.12
In patients with renal dysfunction as evidenced by decreased creatinine clearance, dosage adjustment is appropriate.1
The volume of distribution is about 170 L/m2.
In pediatric patients (ranging from 2-19 years of age) exhibiting relapsed or refractory ALL (acute lymphoblastic leukemia) or AML (acute myelogenous leukemia) receiving a dosage of 52 mg/m2 exhibit a clearance of about 30 L/h/m2.12
Clofarabine is used in the treatment of pediatric patients exhibiting refractory acute lymphoblastic leukemia (ALL) or relapsed ALL following at least two previous treatment approaches.
This agent also increases intracellular levels of cytosine arabinoside with resultant potentiation of ara-C toxicity.
![]() The major
toxicity associated with clofarabine administration is myelosuppression
which increases the likelihood of infectionous complications.
The major
toxicity associated with clofarabine administration is myelosuppression
which increases the likelihood of infectionous complications.
Up to 25% of patients may exhibit a reversible hepatic transaminase enzyme increase.
A number of other side effects have been identified including:
Nausea
Fatigue
Edema and plantar Palmer dysesthesia.
A small number of patients exhibit capillary leak syndrome.
This syndrome presents as hypotension with:
Increased heart rate (tachypnea)
Fever and
Pulmonary edema (x-ray).
![]() Drug
discontinuation is appropriate when patients present with capillary leak
syndrome.
Drug
discontinuation is appropriate when patients present with capillary leak
syndrome.
Electrolyte abnormalities including hypophosphotemia and hypokalemia may also be noted during treatment.
Acute renal injury has been documented in about 30 cases involving refractory/relapse AML being treated using clofarabine.