Medical Pharmacology Chapter 35 Antibacterial Drugs
First Generation Cephalosporins: Cefadroxil
Individual Drug Profiles
Cefadroxil: Audio Overview
Cefadroxil is an orally administered, first‑generation cephalosporin with excellent activity against methicillin‑susceptible staphylococci, streptococci, and a limited spectrum of Gram‑negative bacilli, used primarily for skin and soft‑tissue, urinary tract, and streptococcal pharyngeal infections.8
Cefadroxil exhibits a long elimination half‑life relative to other first‑generation agents which permits once‑ or twice‑daily dosing.7
This characteristic makes cefadroxil particularly appropriate as an attractive oral step‑down option in musculoskeletal and other deep‑seated infections when organisms are susceptible.
|
Cefadroxil Chemistry and Antibacterial Spectrum
Cefadroxil is a semisynthetic first‑generation cephalosporin, structurally related to cephalexin but with a para‑hydroxyphenyl side chain that increases oral bioavailability and prolongs elimination.6
Cefadroxil is bactericidal and targets primarily Gram‑positive cocci, including Staphylococcus aureus (MSSA) and Streptococcus pyogenes, with modest activity against some Enterobacterales such as Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae when β‑lactamase production is limited.4
![]() Clinically
cefadroxil covers typical pathogens in uncomplicated skin and
soft‑tissue infections, lower urinary tract infections, and
streptococcal pharyngitis.2,3,4,9
Clinically
cefadroxil covers typical pathogens in uncomplicated skin and
soft‑tissue infections, lower urinary tract infections, and
streptococcal pharyngitis.2,3,4,9
![]() However, cefadroxil does not reliably cover
methicillin‑resistant S. aureus, enterococci,
anaerobes, or most hospital‑associated multidrug‑resistant
Gram‑negative organisms.
However, cefadroxil does not reliably cover
methicillin‑resistant S. aureus, enterococci,
anaerobes, or most hospital‑associated multidrug‑resistant
Gram‑negative organisms.
This relatively narrow spectrum may be viewed as beneficial since it limits damage to the microbiota compared with broader‑spectrum cephalosporins and fluoroquinolones.
Mechanism of Bactericidal Action
Beta-Lactam Core and Target
Cefadroxil belongs to the β-lactam class of antibiotics.10,12
Bactericidal activity depends on structural integrity of the highly reactive, four-membered β-lactam ring.
![]() The
mechanism of action is the inhibition of bacterial cell wall
synthesis.11
The
mechanism of action is the inhibition of bacterial cell wall
synthesis.11
The bacterial cell wall is a unique and essential structure, one not present in eukaryotic human cells, providing the basis of drug selectivity.
This wall is composed of a rigid, mesh-like polymer known as peptidoglycan, meeting the requirements for structural integrity necessary to maintain the bacterial cell shape and protect it from osmotic lysis due to the hypotonic environment of the human body.13
Covalent Binding to Penicillin-Binding Proteins (PBPs)3,11,14
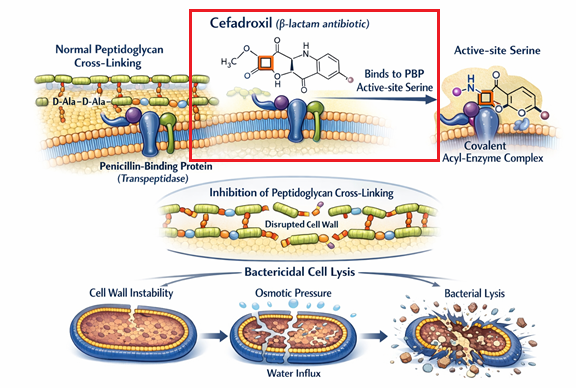 |
Cefadroxil bactericidal activity involves both targeting and inactivating certain bacterial enzymes, the penicillin-binding proteins (PBPs).
These enzymes are anchored on the inner side of the bacterial cell wall and function as transpeptidases.
PBPs are responsible for the final, critical step of peptidoglycan synthesis which is cross-linking of adjacent glycan strands.
Cross-linking is achieved by forming peptide bridges between the D-alanyl-D-alanine termini of adjacent stem peptides.
Cefadroxil, which functions as a structural analog of this natural D-alanyl-D-alanine substrate, possesses a high affinity for the PBP active site.12
Cefadroxil binding to the PBP active site involves acylation forming a stable acyl-enzyme form that irreversible inhibirs the enzyme.
Irreversible inhibition due to acylation prevents the inhibited enzyme from participating in biosynthesis stepsenzymes from the biosynthetic process.
In pathogens such as Streptococcus pneumoniae, cefadroxil has been demonstrated to be an effective inhibitor of multiple essential PBPs, including PBP1a, PBP1b, and PBP2B.
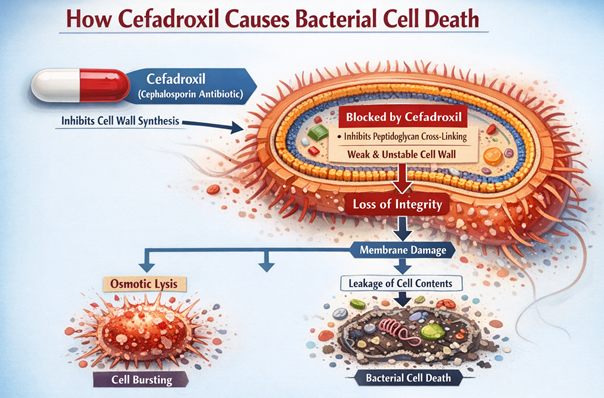
Bacterial Cell Death3,4
By inhibiting the transpeptidase function of PBPs, cefadroxil blocks the third and final stage of bacterial cell wall synthesis.15
This blockade prevents the formation of new, stable cross-links, thereby halting the expansion, repair, and maintenance of the peptidoglycan sacculus.
Inhibition of transpeptidase function of PBPs is particularly consequential in bacteria actively growing and dividing.
Cell division under these circumstances produces defective cross-length cell walls.
Concurrently, bacterial autolytic enzymes, utolysins, that are normally involved in the controlled remodeling and cleavage of the cell wall during growth, continue to function.
Failure of bacterial cell membrane as result of cefadroxil is the combination of both inhibition of cell wall synthesis and continuing autolysis. The cell wall, weakened by these processes, are insufficient to counteract the high internal osmotic pressure of the bacterial cytoplasm, resulting in cell lysis and bacterial death.
Cefadroxil is classified as the bactericidal drug is the result of drug-induced bacterial cell lysis.
 |
|
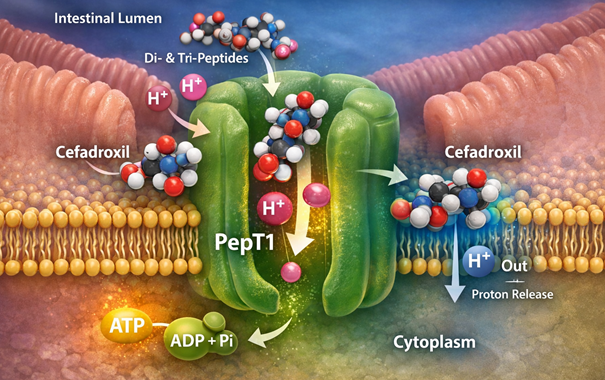
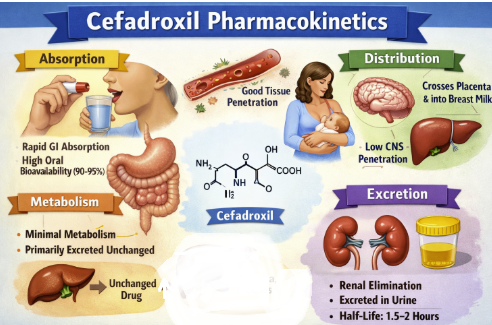
Cefadroxil Absorption3,11
Cefadroxil is rapidly and almost completely absorbed from the gastrointestinal tract following oral administration.
Cefadroxil is noted for its excellent oral bioavailability, likely at least 90%.16
![]() Efficient
uptake is not based on passive diffusion..
Efficient
uptake is not based on passive diffusion..
Cefadroxil, like other aminocephalosporins and aminopenicillins, is a substrate for the high-capacity, proton-coupled intestinal peptide transporter, PepT1 (SLC15A1).17,18
![]() This
transporter, located on the apical membrane of enterocytes in
the small intestine, translocates drug into the portal
circulation, accounting for its high bioavailability.
This
transporter, located on the apical membrane of enterocytes in
the small intestine, translocates drug into the portal
circulation, accounting for its high bioavailability.
 |
|
An important clinical advantage of cefadroxil, distinguishing it from many other oral antibiotics (such as ampicillin or cefaclor), is that its absorption is not significantly affected by the presence of food.
While co-administration with a meal may slightly delay the time to peak concentration (Tmax), the total extent of absorption remain unchanges. This characteristic encourages, patients to take the medication with meals to minimize the common gastrointestinal side effects, without concern for compromising therapeutic efficacy.19
Cefadroxil Distribution
After absorption, cefadroxil is widely distributed throughout the body, into various tissues and fluids.4,20
An important parameter affecting drug distribution is plasma protein binding.4,11,16
Cefadroxil exhibits low protein binding, a highly favorable characteristic.
Cefadroxil is found bound to plasma proteins at a level of about 20% (low).
This low binding is clinically important.
Only the unbound, or "free," fraction of a drug is pharmacologically active and capable of diffusing from the vasculature into the interstitial space to reach the site of infection.
With approximately 80% of the drug in the plasma free and available, cefadroxil can readily achieve effective concentrations at target sites.
Cefadroxil's volume of distribution (Vd) is approximately 0.31 L/kg, suggesting good distribution into the extracellular fluids of the body.4,11,21
Cefadroxil achieves excellent penetration into key infection sites.
The presence of cefadroxil has been described in sputum, muscle, and prostate tissue.
Cefadroxil appears especially effective at penetrating skin-blister fluid, where its penetration appears better than that observed with cephalexin.
Such observations support cephalexin first-line use in skin and skin structure infections (SSTIs).
Cefadroxil crossed the placenta and is distributed in low, but detectable, concentrations into breast milk.
Cefadroxil Metabolism
Cefadroxil exhibits very minimal metabolism.20
![]() Cefadroxil
Excretion 3,4,11
Cefadroxil
Excretion 3,4,11
Cefadroxil is eliminated almost entirely by the kidneys.
Over 90% of an administered dose is excreted unchanged as active, parent drug in the urine within a 24-hour period.
Renal elimination involves two mechanisms.4,23
(1) Glomerular filtration in which the free, unbound fraction of the drug is filtered at the glomerulus.
(2) Active tubular secretion also occurs in which cefadroxil is "pumped" into the tubular lumen by transporters in the proximal tubule.
![]() This
reliance on renal function for elimination means that in
patients with renal impairment, the drug's clearance is severely
compromised, and the drug will accumulate to potentially toxic
levels.
This
reliance on renal function for elimination means that in
patients with renal impairment, the drug's clearance is severely
compromised, and the drug will accumulate to potentially toxic
levels.
 |
December 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |