Medical Pharmacology Chapter 35 Antibacterial Drugs
First Generation Cephalosporins: Cefadroxil
Cefadroxil Pharmacodynamics: The fT > MIC Target 2
As a β-lactam antibiotic, cefadroxil exhibits time-dependent bactericidal activity.
Time -dependent bactericidal activity means its efficacy is not correlated with the peak concentration (CMax) it achieves, but rather with the duration of time that the free drug concentration remains above the minimum inhibitory concentration (fT > MIC) of the target organism.
"fT refers to the percentage of time the free (unbound) concentration of a beta-lactam antibiotic stays above the Minimum Inhibitory Concentration (MIC) of a pathogen during dosing interval, i.e. %fT>MIC)
The typical pharmacodynamic (PD) target for cephalosporins to achieve bacteriostasis and efficacy in most clinical infections is an fT > MIC of 40-50% of the dosing interval.
Here pharmacokinetic and pharmacodynamic relationship is apparent PK/PD integration becomes clear.3,4
Cefadroxil's longer half-life results in a longer fT > MIC for any given dose and MIC value, compared to cephalexin.
Accordingly, cefadroxil may be dosed less frequently compared to cephalexin.
![]() Cefadroxil
Antimicrobial Spectrum
and In Vitro Activity
Cefadroxil
Antimicrobial Spectrum
and In Vitro Activity
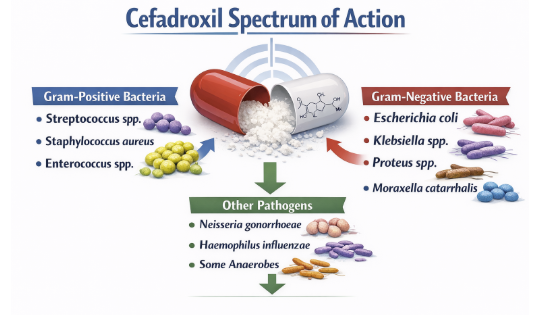 |
As a first-generation cephalosporin, cefadroxil's spectrum of activity is focuses on Gram-positive organisms but exhibits clinically useful activity against a select group of Gram-negative bacilli.5,6,7
![]() Gram-Positive
Anaerobes: Principal Targets for Cefadroxil
Gram-Positive
Anaerobes: Principal Targets for Cefadroxil
 |
|
 |
|
 |
|
![]() Staphylococcus aureus10,11,12
Staphylococcus aureus10,11,12
Cefadroxil demonstrates excellent activity against Methicillin-Susceptible S. aureus (MSSA), which includes strains that produce penicillinase (a beta-lactamase that hydrolyzes penicillin but not cephalosporins).
Cefadroxil remains a first-line oral agent for confirmed MSSA infections.2,9
Streptococcus species:
It is highly active against Streptococcus pyogenes (Group A β-hemolytic streptococci, or GAS), the causative agent of pharyngitis
Cefadroxil is also active against Streptococcus pneumoniae.
In Vitro Potency (MIC Data)2
When its potency is formally tested, cefadroxil activity is confirmed.
For MSSA isolates collected from pediatric patients with musculoskeletal infections, the in vitro activity of cefadroxil was found to be equivalent to that of cephalexin.
Both drugs shared an MIC50 (concentration to inhibit 50% of isolates) of 2 µg/mL and an MIC90 (concentration to inhibit 90% of isolates) of 4 µg/mL.
Gram-Negative Aerobes (Urinary Tract Infections)11,13
Cefadroxil's Gram-negative activity is limited to a few key species, primarily those responsible for uncomplicated urinary tract infections.
Cefadroxil in vitro activity and activity in clinical infections include:
Escherichia coli
Klebsiella species (e.g., K. pneumoniae), and
Proteus mirabilis
|
Skin and Skin Structure Infections (SSTIs)
Pharyngitis and/or Tonsillitis
Urinary Tract Infections (UTIs)
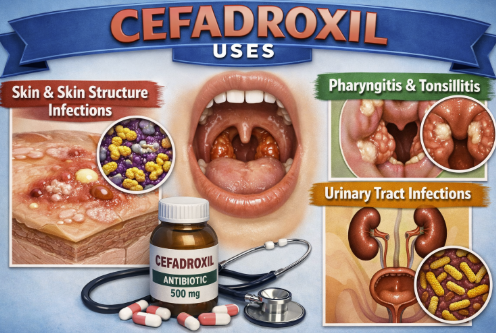 |
Skin and Skin Structure Infections (SSTIs)
Indication
Cefadroxil is indicated for the treatment of mild-to-moderate SSTIs.
These indications include common presentations such as cellulitis, cutaneous abscesses (post-incision and drainage), furunculosis, and impetigo.10,12,13
Pathogens
![]() Cefadroxil
indicated use is specifically for infections caused by
Staphylococci (MSSA) and/or Streptococci, the two
most common pathogens in this setting.
Cefadroxil
indicated use is specifically for infections caused by
Staphylococci (MSSA) and/or Streptococci, the two
most common pathogens in this setting.
Clinical Evidence15,16,17
In appropriate settings, see above, cefadroxil appears to be both they highly effective and reasonable choice.
Its pharmacological profile is ideally suited for this indication, given excellent skin and soft tissue distribution with improved penetration into skin-blister fluid compared to cephalexin.
These factors, along with potent activity against both staphylococcal and streptococcal infection makes cefadroxil a first-line agent for uncomplicated, non-purulent cellulitis (likely streptococcal) or as oral therapy for mild purulent infections (staphylococcal).
Pharyngitis and Tonsillitis (Group A Streptococcus)
Indication10,12,13
Cefadroxil is indicated for the treatment of pharyngitis and tonsillitis caused by Streptococcus pyogenes (Group A β-Hemolytic Streptococci, or GAS).
Clinical Context18
Treatment of pharyngitis and tonsillitis highlight some pharmacological advantages of cefadroxil administration.
Primary goals of treating Group A β-Hemolytic Streptococci pharyngitis involves not only symptomatic improvement, but also complete bacteriological eradication of S. pyogenes from the nasopharynx.
This approach addresses prevention of non-suppurative sequelae of acute rheumatic fever.
Urinary Tract Infections (UTIs)
Indication
Cefadroxil is indicated for the treatment of uncomplicated UTIs (e.g., cystitis) due to susceptible strains of E. coli, P. mirabilis, and Klebsiella species.10,12,13
Clinical Discussion19
The role of cefadroxil in treating UTIs describes evolution of antimicrobial agents of choice.
For many years, first-generation cephalosporins were relegated to second-line status, as result of perceived superiority of trimethoprim-sulfamethoxazole and, more recently, the widespread use of fluoroquinolones.19
Fluoroquinolone-Sparing Approach
The contemporary antibacterial era is defined both by widespread antimicrobial resistance and a new appreciation for antibiotic-associated toxicity.
Amidst the crisis of "bad bugs, few drugs"19 and mounting concerns over fluoroquinolone-driven resistance and their black-box warnings for serious adverse events (e.g., tendon rupture, aortic dissection), cefadroxil is being reevaluated as a highly valuable, narrow-spectrum, fluoroquinolone-sparing alternative.19
The pharmacokinetic profile of cefadroxil appears ideal for treating cystitis.
Cefadroxil is well-absorbed orally, is not metabolized, and administration results in bactericidal concentrations of active drug directly at the site of infection, the urine.
For a confirmed susceptible E. coli cystitis, cefadroxil (or cephalexin) is now considered a preferred, first-line choice.5,19
Adverse Reactions
The most common adverse reactions are gastrointestinal and hypersensitivity.20,21,22
Gastrointestinal adverse effects include:
Diarrhea
Nausea
Vomiting
Abdominal discomfort, and
Hypersensitivity reactions include benign maculopapular rash or urticaria, which are usually mild and reversible upon discontinuation.
Cefadroxil administration has been associated with:
Clostridioides difficile–associated diarrhea ss well as pseudomembranous colitis
These effects may appear during therapy or weeks afterward and should be considered in patients with new‑onset severe or bloody diarrhea.
December 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |